A glance at the history and uses of perk profit in the industry
07 December 2019 sodium hydroxide, chemicals, chemistry articles, the complete history of sodium hydroxide, the most important uses of sodium hydroxide in industry, a comprehensive article about sodium hydroxide, is sodium hydroxide corrosive, the history and uses of sodium hydroxide, the age of sodium hydroxide, the corrosiveness of sodium hydroxide sodium
Perk profit history
An old photo of the first caustic soda tanks in Virginia
An overview of the use and history of sodium hydroxide
The most important uses of sodium hydroxide in industry
We will take a look at the history and uses of sodium hydroxide in the industry, and after examining all aspects of sodium hydroxide, we have an overview of the most important uses of sodium hydroxide in the industry. The product and its low price is required by many industries, for this purpose we decided to present a comprehensive article about sodium hydroxide or solid sodium hydroxide. Stay with Perk Chemi to check this practical and valuable material from all aspects.
Reading the following article helps to better understand the issue:
Why interest perk?
Profit perk | Caustic soda Solid sodium hydroxide Caustic soda flakes
What you will read in this article:
• Perk profit history
• Sodium hydroxide production methods
• Applications of sodium hydroxide
• Use of perk profit in different purposes
• Safety information
• Conclusion
Introduction
Sodium hydroxide or caustic soda is a white and odorless crystalline substance, this substance is considered a strong base and is also known as sodium hydrate. Sodium hydroxide exists in solid and liquid form, solid sodium hydroxide is produced in the form of flakes, powder and granules. Depending on its appearance and purity, it is used in different industries. Its granule usually has a higher purity, so it is used in more sensitive industries such as pharmaceuticals.
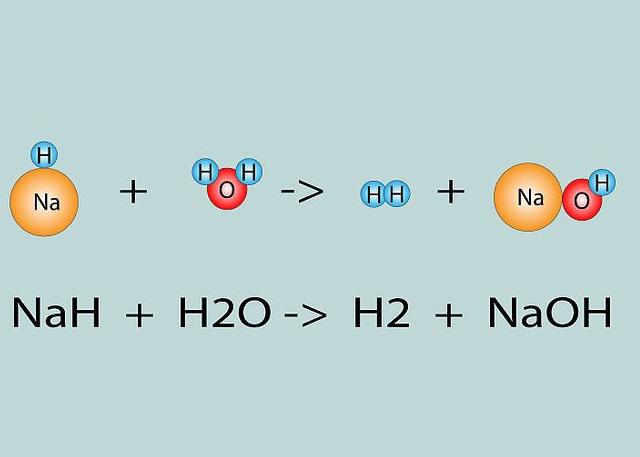
The reaction of absorbing moisture by caustic soda is very exothermic and therefore causes water to splash around, so we must observe safety precautions when working with it. Sodium hydroxide reacts with some metals such as aluminum and produces hydrogen gas, it also reacts with sulfur dioxide and eliminates the effect of producing toxic and harmful gases such as H2S and SO2, which are usually obtained from burning coal. The result of burning soda can prevent the release of these toxic gases in the atmosphere.
Corrosivity of sodium hydroxide
Is sodium hydroxide corrosive? Yes, sodium hydroxide is very corrosive, its corrosiveness is very high on aluminum and zinc, and it is slightly corrosive to lead and tin, but it does not affect other metals. Sodium hydroxide is widely used in the synthesis processes of organic compounds, in refineries, textile industries, paperboard making, soap making, aluminum production, food industries, etc. The main purpose of producing and using this substance is primarily to benefit from its degreasing properties. is.
History of Sood Perak
About the age of Sood Perak, it should be stated that sodium hydroxide was discovered by English scientist Humphrey Day in 1807. It is interesting to know that the incident or experiment that led to the discovery of sodium hydroxide remains unknown. For some time, until a few years after its discovery, this substance was known as a primary substance called alkali. It should be noted that sodium hydroxide was used long before its identification. At the time of the ancient Egyptians and Babylonians, a weak solution of this substance was produced by using vegetable ash with water. One of the important historical evidences that tells about the age of this material is a mixture of alkaline water with olive oil to produce a product that was used on a clay tablet about 4800 years old.
Sodium hydroxide production methods
Sodium hydroxide or caustic soda is considered a strong base and is generally produced as a solid or 5% solution, since this material is cheap, it is widely used and mostly as a chemical and It is used in the manufacture of other chemicals. The main use of sodium hydroxide is degreasing. Sodium hydroxide with fats creates a soluble substance that can be dissolved in water, hence the degreasing is very strong.
The main use of sodium hydroxide is degreasing.
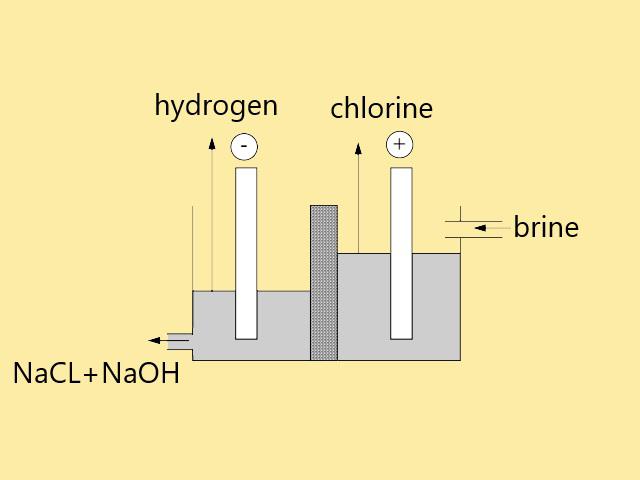
Types of sodium hydroxide production methods
The main method for the production of sodium hydroxide is the electrolytic separation of sodium, from the electrolysis of a concentrated solution of sodium chloride, chlorine gas, hydrogen gas and sodium hydroxide solution are produced (the common products of electrolytic production are caustic soda, chlorine and hydrogen). Sodium hydroxide is collected in the cathode. . Where water is converted into hydrogen gas and hydroxyl ions and the diluted caustic salt leaves the cell (negative electrode). Chlorine gas is also produced in the anode (positive electrode).
(2Nacl(aq)+2H2O(l)→H2(q)+CL2(q)+2NaOH(aq
3 types of electrolytic cell are used to produce sodium hydroxide from salt:
(Mercury Process) Castner-Kellner Cell
Nelson Diaphragm Cell
Membrane Cell
Almost all caustic soda is produced by electrolysis of sodium chloride solution using one of the 3 types of cells mentioned. The raw material used is common salt, sodium chloride solution is often called brine, traditionally, electrolysis is performed by mercury amalgam diaphragm cell, but ion exchange membrane cell is more environmentally and economically is used. In the United States, diaphragm cells are used, and in Europe, mercury and membrane cells are used. In the mercury cell, sodium is discharged in the form of sodium amalgam ions of mercury and chloride in the form of chlorine.
Amalgam is transferred to a completely separate chamber where it reacts with water and produces sodium hydroxide solution and hydrogen gas. The presence of chlorine and mercury gas will cause air pollution, but the product obtained from this method is very pure and will have a high quality. The diaphragm cell is usually made of asbestos, it passes the salt water flow from the anode to the cathode, but it separates the space of chlorine gas and hydrogen gas. By discharging hydrogen ions, hydroxide ions in the cathode chamber with sodium ions. Blue collects and produces sodium hydroxide. The migration of hydroxide ions from the cathode to the anode is prevented by the liquid flow rate from one chamber to another.
The chlorine formed in the anodes increases through salt water to a space formed by the cover cell, but the finished product will have a lower grade due to the presence of impurities. In the membrane process, the ion exchange membrane acts as a barrier against all currents. It works as a gas and liquid and only allows sodium ions to pass through the chambers. Sodium ions pass in hydrated form and sodium hydroxide is produced in the cathode where hydrogen is released, chlorine gas is released in it. will be
The membrane is a copolymer, tetrafluoroethylene or a similar fluorine monomer. Mercury cells are cheaper than diaphragm cells, they cost less electricity and a product with high concentration and purity is produced, but the mercury must be removed from the effluent. Diaphragm cells require a large amount of heat energy to produce a more concentrated solution. can be achieved, but if there is little steam and structure, they can be cheaper than mercury cells. The use of membrane cells is growing due to the reduction of capital and energy costs and the absence of environmental problems. There is another way to produce There is sodium hydroxide with low purity, in this method Trona stone, which is a mixture of sodium carbonate with lime, is used to produce sodium hydroxide.
Chemical and physical properties of sodium hydroxide
chemical formula : NaOH
Boiling point: 1390ºc
density : gr/ml 2.13
Molecular weight : 39.99 gr/mol
Similar names: Caustic soda, sodium hydrate, soda lye
Solubility: Soluble in water, ethanol and glycerol and insoluble in acetone and ether
Sodium hydroxide is a white, odorless, crystalline solid that absorbs moisture from the air, when dissolved in water or neutralized with acid it releases considerable heat which may be sufficient to ignite combustible materials. Sodium hydroxide is very corrosive and is generally used as a solid or 50% solution. It is a strong base and readily reacts with acids such as hydrochloric acid to form the corresponding salts.
NaOH+HCL→NaCl+H2O
Caustic soda is very corrosive to aluminum and zinc and has little corrosion to lead and tin and does not affect other metals.
Sodium hydroxide has good reactivity with metals and acids. For example, sodium hydroxide can be used to remove dangerous gases such as sulfur dioxide (SO2). Also, superperc can be used to precipitate metals in the form of hydroxide from water.



